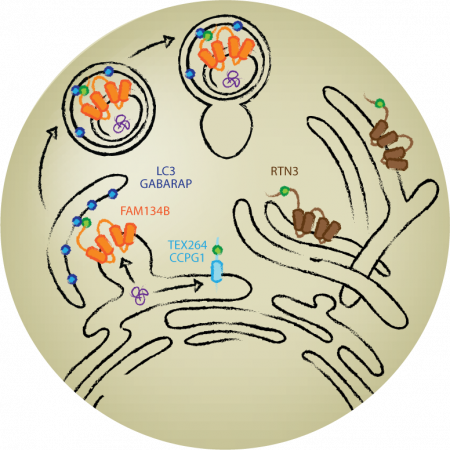
ER-phagy involves selective degradation of endoplasmic reticulum (ER), which is dynamically remodelled to adapt its structure to cellular needs. We have previously shown that ER-phagy is regulated by a subset of reticulon-type receptors, namely FAM134B and RTN3, which was followed by the discovery of three additional ER-phagy receptors, Sec62, CCPG1 and TEX264. These ER-phagy receptors exhibit different preferences in localization at the specific ER subdomains, the cargo selectivity and remodeling of the ER architecture. Yet, the molecular mechanisms underlying ER-phagy receptor action, activation or selectivity for ER remodelling upon cellular stress, remain unexplored.
Our lab focuses on – (1) understanding the function and regulation of ER-phagy receptors and their binding partners, with particular interest in ER Chaperones; (2) understanding the underlying mechanisms of the delivery of portions of ER to the lysosome, mainly through identification of novel co-receptors required for structure support, sensing, scission and the delivery of ER fragments to the lysosome. To address these areas of interest, we are utilizing biochemical methods, mass spectrometry and CRISPR/Cas9 screening in collaboration with FCSC laboratory within our institute. We are utilizing mouse models to investigate the implications the impairment in ER-phagy pathway has in vivo and in a physiological context.
 CRISPRi
CRISPRi PROTEOMICS
PROTEOMICS MICROSCOPY
MICROSCOPY VIRUS
VIRUS CANCER
CANCER NEURODISEASE
NEURODISEASE BACTERIA
BACTERIA