Proteostasis
Areas of Research

mtUPR
Mitochondrial function is of central importance for cells. We study how cells respond to mitochondrial protein misfolding via the mitochondrial unfolded protein response (mtUPR).
Autophagy / Mitophagy
Clearance of damaged cellular components plays a critical role in cellular function. We study these mechanisms to get new insights into disease mechanisms.
Cellular Stress Responses
Our cells can respond to different stressors to adapt to the changing environment. Depending on source of the stress the responses differ. We aim to understand the regulation, interactions and outcomes of cellular stress responses.
mtUPR: Mitochondrial UPR
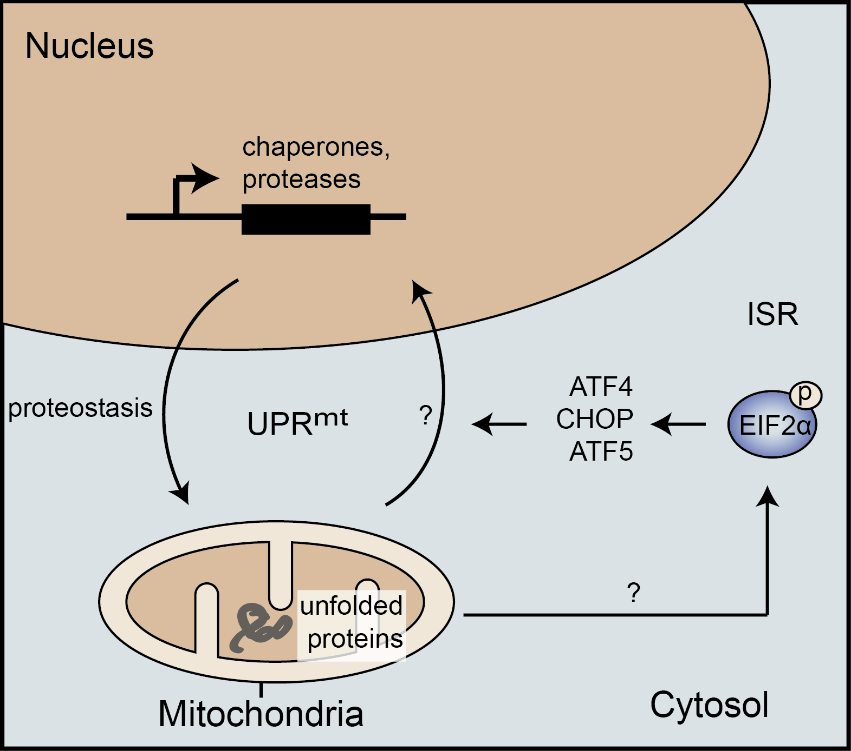
mtUPR: Unfolded proteins inside mitochondria
Mitochondrial homeostasis is crucial for cell viability. As in other cellular compartments, protein homeostasis (proteostasis) in mitochondria is maintained by a complex network controlling protein synthesis, import, processing, folding, and degradation. Upon mitochondrial protein misfolding, the mitochondrial unfolded protein response (mtUPR) activates to induce a specific transcriptional response leading to an increase in the folding capacity, particularly via mitochondrial chaperonins (reviewed in Münch, BMC Biology, 2018). While the mtUPR is well studied and characterized in C. elegans, our understanding of the signaling and molecular mechanisms underlying the mtUPR in mammalian cells remains lacking.Adding to the complexity of studying the mammalian mtUPR, a wide range of mitochondrial stresses not causing protein misfolding lead to activation of the integrated stress response, but not mtUPR markers (Münch and Harper, Nature 2016). Chaperonin-induction remains specific to the response to protein unfolding. This observation shows a central role of the integrated stress response to respond to various mitochondrial stresses in addition to numerous cellular stresses. However, it also reveals that there appears to be a specific mtUPR signaling that relays information on mitochondrial proteostasis to a specific transcriptional output, including chaperonin induction.
Münch C, Harper JW, Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation. Nature 2016. 534 (7609) 710-3.
Klann K, Tascher G, Münch C, Functional Translatome Proteomics Reveal Converging and Dose-Dependent Regulation by mTORC1 and eIF2α. Mol Cell 2020. 77 (4) 913-925.e4
Münch C, The different axes of the mammalian mitochondrial unfolded protein response. BMC Biol 2018. 16 (1) 81
Michaelis JB et al., Protein import motor complex reacts to mitochondrial misfolding by reducing protein import and activating mitophagy. Nature Communications 2022. 13 (1) 5164
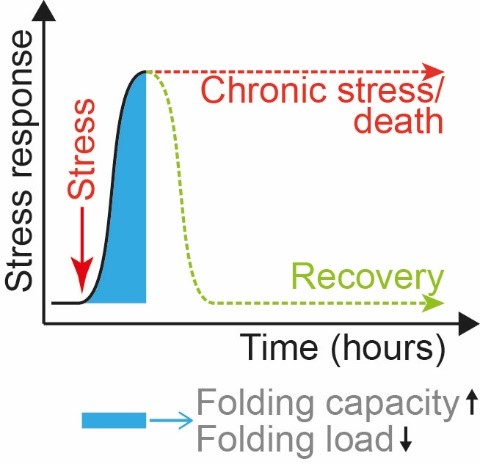
Timing: The transient nature of stress responses
One major aspect in studying stress responses is their highly transient nature, typically lasting several hours with chronic activation leading to compensation and cell death. Thus, to investigate acute responses to unfolded mitochondrial proteins, we utilize chemical inhibitors that rapidly induce protein misfolding to allow a time-resolved study of the resulting signaling and cellular modulation. Using these tools, we recently defined the acute transcriptional mtUPR and discovered a novel mtUPR axis leading to a decrease in mitochondrial RNA processing and translation. This pathway provides with an additional approach to restore mitochondrial (and cellular) proteostasis.
Autophagy / Mitophagy
Autophagy: Removing dysfunctional components and organelles
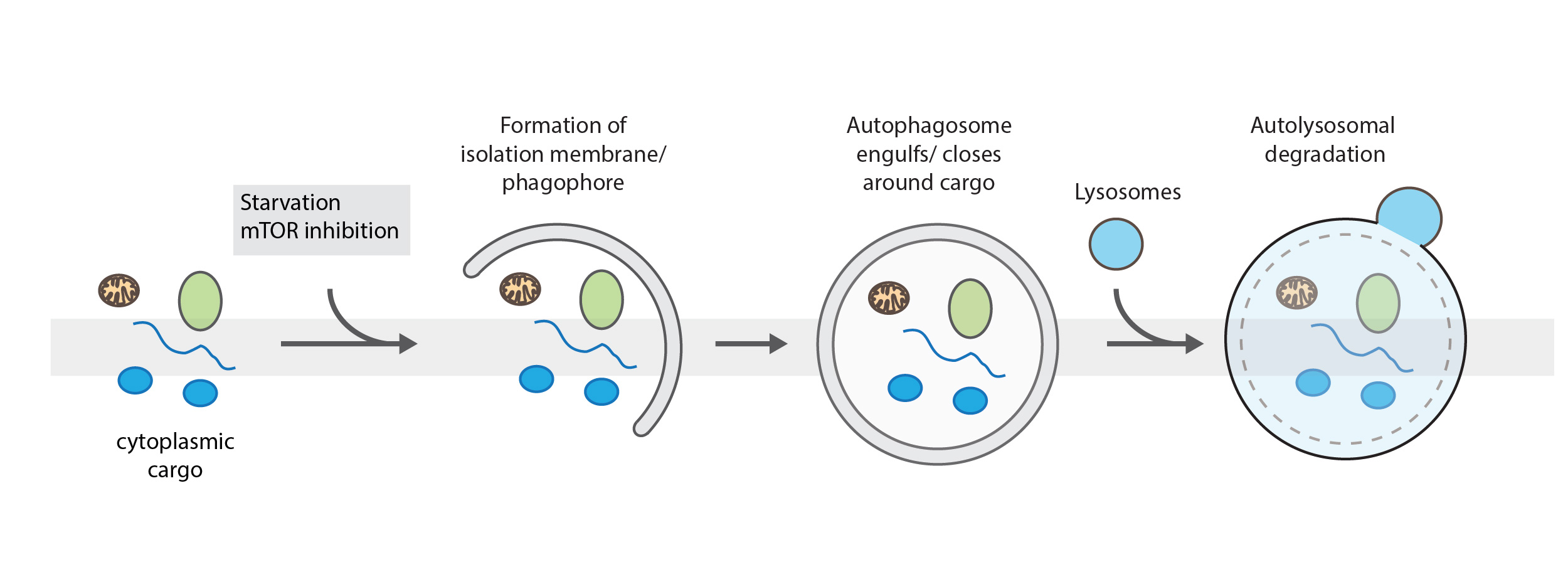
Two distinct pathways for autophagy have been described. Bulk autophagy, which is activated in states of starvation and selective autophagy, when damaged organelles or large defective structures have to be degraded. It is a highly regulated process to remove damaged cellular content or provide energy and building blocks. For both pathways an isolation membrane, the phagophore expands and engulfs cargo in a double membrane structure, called autophagosome. Then, autophagosomes fuse with lysosomes leading to the degradation of their content. In bulk autophagy, cytoplasmic content is enclosed in a non-specific manner. During selective autophagy, specific cargo, such as damaged mitochondria (mitophagy), pathogens (xenophagy) or protein aggregates (aggrephagy) are marked and sequestered into autophagosomes for their degradation.
C. Poluzzi, M. V. Nastase, J. Zeng-Brouwers, H. Roedig, L. T. H. Hsieh, J. B. Michaelis, E. M. Buhl, F. Rezende, Y. Manavski, A. Bleich, P. Boor, R. P. Brandes, J. Pfeilschifter, E. H. K. Stelzer, C. Münch, I. Dikic, C. Brandts, R. V. Iozzo, M. Wygrecka, L. Schaefer, Biglycan evokes autophagy in macrophages via a novel CD44/Toll-like receptor 4 signaling axis in ischemia/reperfusion injury. Kidney Int. 95, 540–562 (2019)
N. Meyer, L. Henkel, B. Linder, S. Zielke, G. Tascher, S. Trautmann, G. Geisslinger, C. Münch, S. Fulda, I. Tegeder, D. Kögel, Autophagy activation, lipotoxicity and lysosomal membrane permeabilization synergize to promote pimozide- and loperamide-induced glioma cell death. Autophagy (2021)
Mitophagy: Clearance of damaged mitochondria
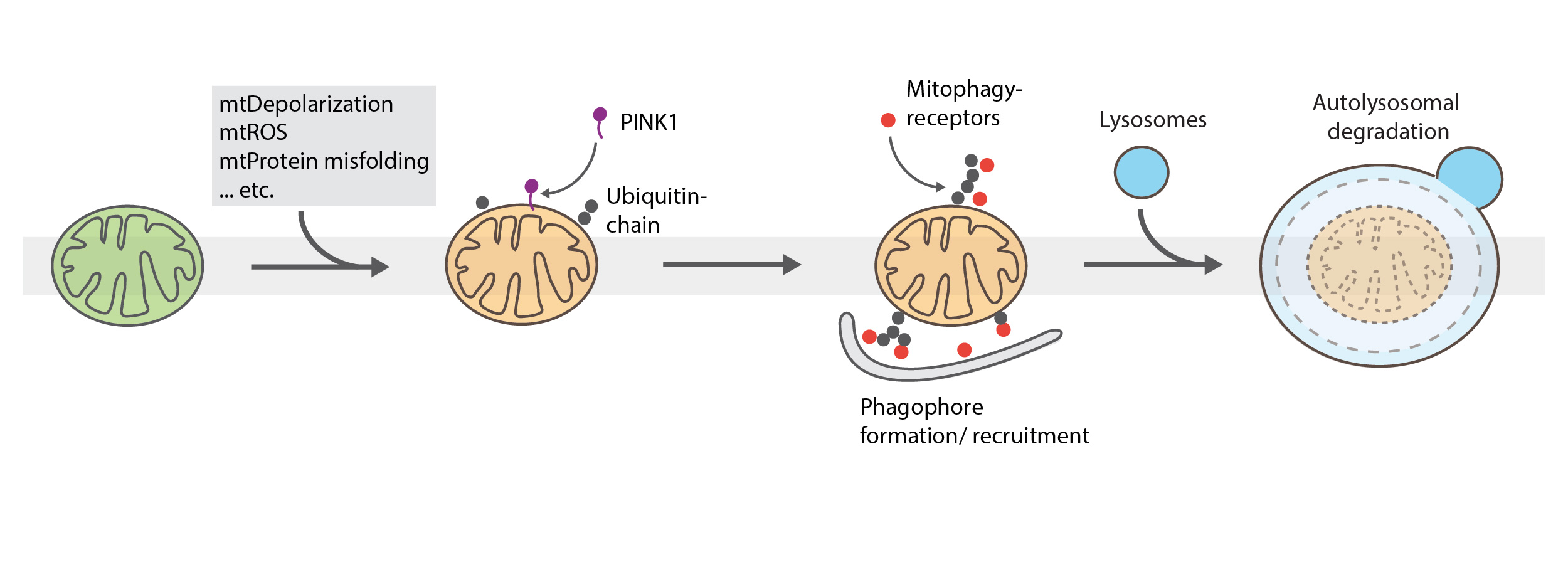
Mitophagy is the selective autophagy of mitochondria and plays a critical role in mitochondrial health, cell viability, removal of sperm-derived mitochondria after fertilization and several neurological disorders. The canonical mitophagy pathway is driven by PINK1 accumulation upon mitochondrial depolarization, poly-ubiquitination via Parkin and recruitment of mitophagy-receptors to the mitochondrial surface. The isolation membrane/phagophore is formed around the marked organelle followed by enzymatic degradation in the autolysosome.
We investigate mitophagy induction and its downstream pathways using quantitative proteomics, CRISPR/Cas9 gene editing, fluorescent activated cell sorting and quantitative immunoblotting and microscopy. Our main goals are to understand how mitophagy activates upon mitochondrial protein misfolding and what are the functional links to the mitochondrial unfolded protein response.
N. Meyer, S. Zielke, J. B. Michaelis, B. Linder, V. Warnsmann, S. Rakel, H. D. Osiewacz, S. Fulda, M. Mittelbronn, C. Münch, C. Behrends, D. Kögel, AT 101 induces early mitochondrial dysfunction and HMOX1 (heme oxygenase 1) to trigger mitophagic cell death in glioma cells. Autophagy. 14, 1693–1709 (2018)
K. Yamano, C. Wang, S. A. Sarraf, C. Münch, R. Kikuchi, N. N. Noda, Y. Hizukuri, M. T. Kanemaki, W. Harper, K. Tanaka, N. Matsuda, R. J. Youle, Endosomal rab cycles regulate parkin-mediated mitophagy. Elife. 7 (2018)
D. Nguyen, S. Shaid, O. Vakhrusheva, S. E. Koschade, K. Klann, M. Thölken, F. Baker, J. Zhang, T. Oellerich, D. Sürün, A. Derlet, I. Haberbosch, S. Eimer, H. D. Osiewacz, C. Behrends,C. Münch, I. Dikic, C. H. Brandts, Loss of the selective autophagy receptor p62 impairs murine myeloid leukemia progression and mitophagy. Blood. 133, 168–179 (2019)
Cellular Stress Responses
Cells continuously adapt to altering environmental conditions to maintain cellular homeostasis. External stimuli, such as small molecules, pathogenic bacteria and viruses, can trigger cellular stress responses. These dedicated pathways include specific molecular signaling pathways and organelle-specific protein quality control mechanisms to overcome damage.
RNF213 is a giant protein composed of a unique combination of AAA+ ATPase and E3 ubiquitin ligase. It is widely known for its affiliation with Moyamoya disease. Recently, RNF213 emerged as a novel player in host cell response to pathogenic stress. We are focusing on the characterization of the molecular and biochemical function of RNF213 to better understand its role in cellular homeostasis and disease.
Mitochondria, the central hub for cellular metabolism, plays a pivotal role in modulating stress signaling to maintain cellular homeostasis and dynamically adapt to changing energy requirements of the cell. Altered mitochondrial activity or mitochondrial dysfunction can trigger accumulation of senescent cells, which has severe implications in organismal ageing and age-associated diseases. However, the molecular signaling and regulatory events underlying mitochondrial stress induced senescence remain elusive. We aim to decipher the molecular mechanisms regulating mitochondrial stress signaling during senescence.
Mitochondrial RNA granules (MRG) are large membrane less structures essential for multiple steps of mitochondrial RNA metabolism. Despite the fact that mutations in components of those granules are linked to neurological disorders, the overall composition, structure and especially dynamic properties upon stress are unknown. We employ proximity labelling combined with quantitative mass spectrometry to elucidate the MRG architecture at high depth and to characterize their dynamic regulation upon stress.
The endoplasmic reticulum (ER) is the central hub for the secretion of proteins and faces a high amount of nascent proteins that require correct folding. We explore how cells adapt their ER protein quality control to various cellular stresses. Protein folding per se is error-prone and cells developed an elegant system for sensing and resolving accumulating amounts of unfolded and misfolded proteins, collectively known as the unfolded protein response (UPR). Mammalian cells contain three parallel UPR branches with different functions that cooperatively remodel the cellular proteome such that the folding deficit in the ER is overcome. We seek to understand how protein import at the ER is affected under stress conditions and define the contribution of the individual UPR branches in this scenario. By combining molecular, genetic and biochemical assays along with multi-layered systems biology, we aspire to unravel organellar and cellular stress signaling mechanisms to reveal their role in human diseases.