mtUPR: Unfolded proteins inside mitochondria
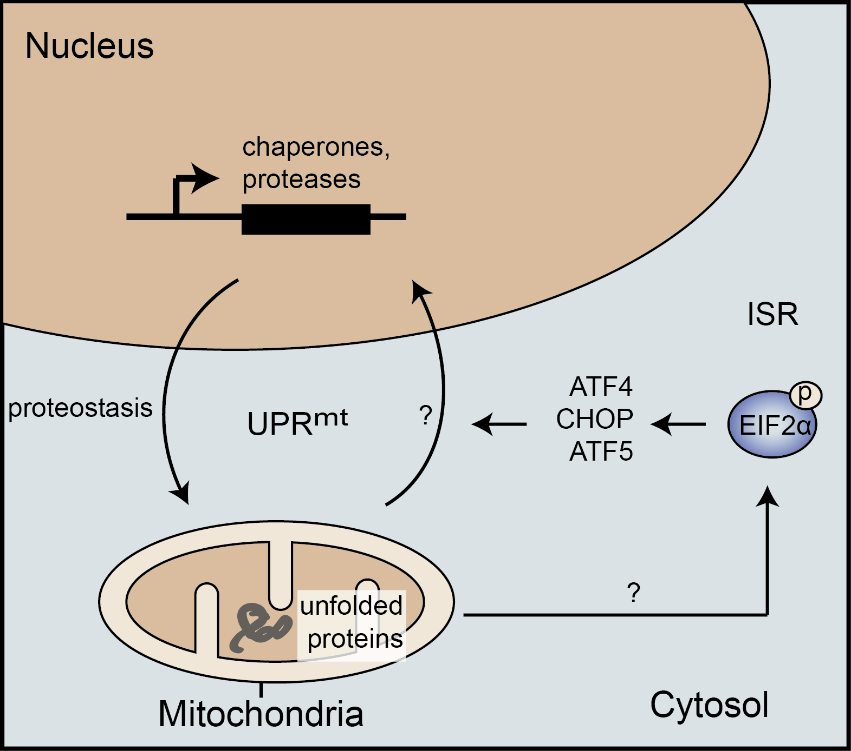
Mitochondrial homeostasis is crucial for cell viability. As in other cellular compartments, protein homeostasis (proteostasis) in mitochondria is maintained by a complex network controlling protein synthesis, import, processing, folding, and degradation. Upon mitochondrial protein misfolding, the mitochondrial unfolded protein response (mtUPR) activates to induce a specific transcriptional response leading to an increase in the folding capacity, particularly via mitochondrial chaperonins (reviewed in Münch, BMC Biology, 2018). While the mtUPR is well studied and characterized in C. elegans, our understanding of the signaling and molecular mechanisms underlying the mtUPR in mammalian cells remains lacking.Adding to the complexity of studying the mammalian mtUPR, a wide range of mitochondrial stresses not causing protein misfolding lead to activation of the integrated stress response, but not mtUPR markers (Münch and Harper, Nature 2016). Chaperonin-induction remains specific to the response to protein unfolding. This observation shows a central role of the integrated stress response to respond to various mitochondrial stresses in addition to numerous cellular stresses. However, it also reveals that there appears to be a specific mtUPR signaling that relays information on mitochondrial proteostasis to a specific transcriptional output, including chaperonin induction.
Münch C, Harper JW, Mitochondrial unfolded protein response controls matrix pre-RNA processing and translation. Nature 2016. 534 (7609) 710-3.
Klann K, Tascher G, Münch C, Functional Translatome Proteomics Reveal Converging and Dose-Dependent Regulation by mTORC1 and eIF2α. Mol Cell 2020. 77 (4) 913-925.e4
Münch C, The different axes of the mammalian mitochondrial unfolded protein response. BMC Biol 2018. 16 (1) 81
Michaelis JB et al., Protein import motor complex reacts to mitochondrial misfolding by reducing protein import and activating mitophagy. Nature Communications 2022. 13 (1) 5164