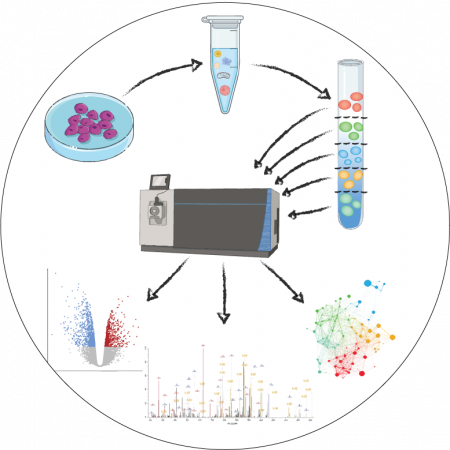
Alteration of biological processes caused by pathological, genetic and environmental factors can result in dynamic changes in the proteome and post-translational modifications (PTMs). Most of the studies in our lab rely on cutting-edge proteomics techniques. Several of our research projects rely on proteomics as a major tool. We are interested in subcellular proteomics, we look for changes in organelle proteome, ubiquitinome and phosphoproteome in response to specific stressors or in knock out (KO) cell-lines. Using lysosomal Lys-ϵ-Gly-Gly remnant proteomics, we have identified several lysosomal membrane proteins that are modified by ubiquitination under specific stress conditions. Daily, we perform proteomics in search of novel interaction partners of proteins of interest, as well as differences in protein levels upon applied conditions.
 PROTEOMICS
PROTEOMICS