News from the Institute
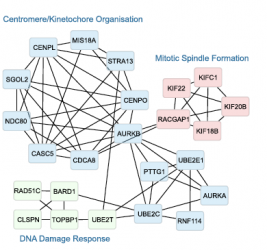
Defects in SUMOylation have long been suspected as potential drivers of carcinogenesis, however, positive proof has been lacking. The post-translational modification of proteins with the ubiquitin-like modifier SUMO is known to regulate numerous essential cellular functions, and aberrant SUMO patterns have frequently been found in cancers. Now, a team of researchers around IBC2 Vice Director Stefan Müller and Ulrich Keller from Charité has identified the missing mechanistic link: They identified a tumor suppressing function for SUMO deconjugase SENP6, one of the enzymes responsible for reversion of SUMO modifications. In one third of B-cell lymphoma patients, SENP6 was found mutated or deleted. Loss of SENP6 was then in a next step shown to directly affect DNA repair and genome maintenance by destabilizing protein complexes involved in both essential pathways, ultimately leading to genome instability.
... (read more)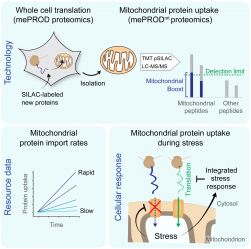
The great majority of mitochondrial proteins is encoded on the nuclear genome. As a consequence, translation of these proteins in the cytosol is followed by their translocation into mitochondria. Compromised mitochondrial protein import has been linked to various pathologies. Up to this point, investigation of protein import into organelles remained challenging due to a lack of suitable tools. The team around Christian Münch developed a proteomics-based assay that allows examining mitochondrial protein uptake at the global scale in unperturbed cells.
... (read more)
Christian Münch, group leader at the Institute of Biochemistry II of Goethe University Frankfurt, was selected as an EMBO Young Investigator. The EMBO Young Investigator Program recognizes and supports life scientists, who have already demonstrated scientific excellence and are within the first years of their independent group. It provides with financial support, access to the European Molecular Biology Laboratory (EMBL) core facilities, as well as a variety of training and networking opport
... (read more)
The Academy of Sciences and Literature in Mainz has welcomed 13 new members in their ranks, among them IBC2 Director Ivan Đikić. Đikić was in prominent company – BioNTech founders Uğur Şahin and Özlem Tureci were also amongst the new members announced. The Mainz Academy is one of Germany’s eight academies of science, its objectives are the cultivation of sciences and literature as well as preservation and promotion of culture. It is divided into three Classes: Mathematics and Natural Sciences, Humanities and Social Sciences, Literature and Music.
... (read more)
The list of Highly Cited Researcher identifies pioneers in their respective fields who repeatedly demonstrated significant and broad influence. IBC2 Director Ivan Đikić is honored for generating exceptional impact by publishing multiple highly cited papers in the past decade in even two fields, “Biology and Biochemistry” and “Molecular Biology and Genetics”. His papers in these fields rank in the top 1% by citations. The analysis is based on citations as recorded in Web of Science, for 2021 all publications between 2010 and 2020 were considered.
... (read more)