22 Dec 2020 - Essential role of accessory subunit LYRM6 in the mechanism of mitochondrial complex I.
IBC2 research fellow Heike Angerer found a key role of accessory subunit LYRM6/NDUFA6 in energy conversion by eukaryotic respiratory complex I. These results have been recently published in Nature Communications.
In an integrated approach combining biochemistry, structural biology and multiscale molecular simulations, researchers from IBC2 and from the group of Vivek Sharma at the Department of Physics, University of Helsinki, found that specific interactions of LYRM6/NDUFA6 with core subunits are indispensable for the function of mitochondrial complex I.
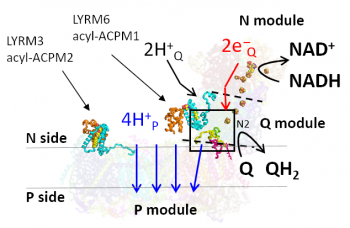
Respiratory complex I (NADH:ubiquinone oxidoreductase) is the entry point of electrons from NADH into the respiratory chain. In contrast to the bacterial enzyme with 14 central subunits, mitochondrial complex I contains up to 30 additional so-called accessory subunits. Accessory subunits of complex I were suggested to stabilize and regulate the giant proton pump but their role has remained poorly understood. The characterization of core and accessory subunits is important to determine the role of mitochondrial complex I in neurodegenerative and ageing-related diseases.
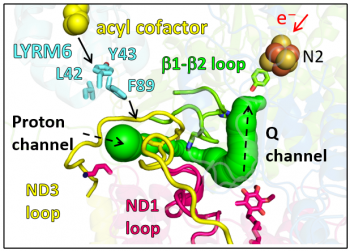
Here, the team led by Heike Angerer and Vivek Sharma found that accessory subunit LYRM6/NDUFA6 not only plays a role in structural stabilization, but is also involved in core energy conversion reactions of complex I. Molecular modeling and multi-scale simulations of wild type and an inactive LYRM6 mutant identified a putative proton transfer pathway through which protons required for substrate reduction can be transferred, but it was found to be blocked due to structural perturbations in the mutant.