News from the Institute
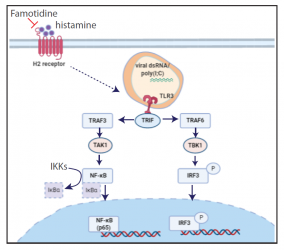
Until now, treatment options for COVID-19 patients are rather limited. A poor disease outcome is known to be linked to an uncontrolled inflammatory response, triggered by a cytokine storm and leading to wide-spread inflammation, severely damaging the lung and other organs. An interdisciplinary team around IBC2 Director Ivan Đikić now reports that inflammatory signaling activated by SARS-CoV-2 can be inhibited by a drug named famotidine. The drug acts as H2 receptor antagonist and is widely used for treatment of heartburn and prevention of stomach ulcers. Early on in the pandemic, retrospective studies have drawn the interest towards this drug, as it apparently reduced the risk of intubation and death for patients being hospitalized for COVID-19.
... (read more)
Santosh Kumar Kuncha was awarded with one of the prestigious Research Fellowships from the European Molecular Biology Organization (EMBO), which enable doctoral researchers from abroad to pursue their postdoctoral research in Europe and around the world
... (read more)
As announced today, the PROXIDRUGS consortium led by IBC2 Director Ivan Đikić has been selected for funding within the Clusters4Future initiative of the Federal Ministry of Education and Research (BMBF), which aims at promoting regional innovation networks for the benefit of economy and society. PROXIDRUGS will be funded with up to 15 M € for the next three years, as only one of 7 clusters selected for funding from a total of 137 applications
... (read more)
As announced today, scientists from Goethe University Frankfurt were successful in securing funding for the ENABLE cluster project which aims at identifying disease-relevant key targets and enabling innovative therapeutic strategies. For the next four years, the project will be supported with 8 M€ by the State of Hesse as part of the cluster initiative to prepare for the next round of the federal Excellence Strategy. The ENABLE research program is centered around inflammation, infection and cellular homeostasis, which are all impacting on a broad range of diseases, many of them with high unmet medical need. It relies on innovative technologies and close team work between basic and translational research.
... (read more)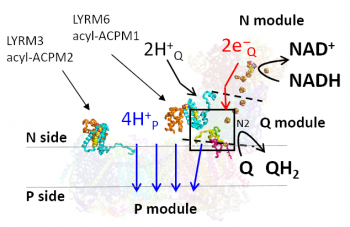
IBC2 research fellow Heike Angerer found a key role of accessory subunit LYRM6/NDUFA6 in energy conversion by eukaryotic respiratory complex I. These results have been recently published in Nature Communications.
In an integrated approach combining biochemistry, structural biology and multiscale molecular simulations, researchers from IBC2 and from the group of Vivek Sharma at the Department of Physics, University of Helsinki, found that specific interactions of LYRM6/NDUFA6 with core subunits are indispensable for the function of mitochondrial complex I.