14 Nov 2024 - International collaboration discovers role of ubiquitination and ER phagy in combatting defective splicing
In a study published in today’s issue of Science, a team around IBC2 Director Ivan Đikić reveals how cells deploy ubiquitination and selective autophagy to combat the harmful effects of cryptic splicing, protecting against accumulation of misfolded proteins and preserving cellular health.
Splicing is a precisely regulated process facilitated by the spliceosome, a large protein complex composed of more than 200 proteins. Defects in this dynamic process cause diseases such as retinitis pigmentosa (RP), a genetic disorder that leads to progressive retina degeneration due to aberrant splicing in photoreceptor cells.
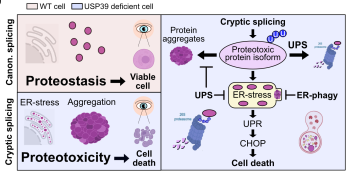
The team around Đikić now discovered a novel mechanistic link between the deubiquitinase USP39 and impaired RNA splicing. Using zebrafish and cellular models, they demonstrated that USP39 depletion mimics RP-like phenotypes with retinal degeneration. USP39 deficiency led to activation of cryptic 5’ splice sites, resulting in the production of aberrant protein isoforms that are prone to misfolding and aggregation. The misfolded proteins and protein aggregates disrupt cellular proteostasis and trigger cellular stress responses.
The team analyzed the repertoire of stress responses following USP39 depletion and found that cells ramped up ubiquitination and ER-phagy, a specialized form of selective autophagy targeting the endoplasmic reticulum (ER). By this, misfolded proteins can be removed and ER stress alleviated. If the proteotoxic burden cannot be successfully managed, then this leads to proteotoxic stress, prolongation of ER stress, and, ultimately, apoptotic cell death.
“To uncover the dynamic changes in the components of the spliceosome that can transform normal splicing into defective cryptic splicing, we had to apply a wide range of multidisciplinary approaches”, explained Cristian Prieto-Garcia, postdoctoral researcher and first author of the study.
“Mathematical and computational methods allowed us to monitor cryptic splicing, while molecular and biochemical studies revealed how spliced proteins generate toxic aggregates that eventually lead to cell death and cause a retinitis-like syndrome in the zebrafish model”, said Ivan Đikić, who brought the international team together that comprised members from several Goethe University departments, from the University of Stuttgart, the IMB Mainz, the two MPIs of Biophysics and for Heart and Lung Research, the EMBL in Grenoble, and from Chinese as well as Japanese Universities.
The result highlights the central role of the ubiquitin and autophagy systems in the pathology of spliceosome-associated diseases such as RP. They also suggest that enhancing protein degradation pathways could serve as a therapeutic approach to mitigate the toxic effects of cryptic splicing associated with multiple diseases. Ivan Đikić emphasized the far-reaching impact of this study: “This discovery opens up new avenues of research, and one of the most exciting is the targeting of aggressive, highly proliferative cancer cells that are dependent on high splicing rates”.