21 Feb 2024 - Targeted protein degradation via intramolecular bivalent glues
A new class of molecular glue could pave the way for a new generation of drugs to target cancers and neurodegenerative diseases.
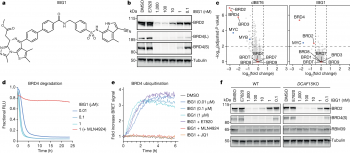
Targeted protein degradation (TPD) is an emerging field of drug development for treating diseases that involves redirecting protein recycling systems in our cells to destroy disease-causing proteins. Most TPD strategies use small molecules - so-called degraders - to recruit these target proteins to a class of enzymes called ubiquitin E3 ligases. The E3 tags the target protein with ubiquitin labels, which ultimately leads to the destruction of the disease-causing protein via the cellular waste bin: the proteasome.
Research teams at the University’s Centre for Targeted Protein Degradation (CeTPD) led by Professor Alessio Ciulli and at the Research Center for Molecular Medicine (CEMM) of the Austrian Academy of Sciences led by Dr. Georg Winter together with collaborators from Eisai Co. Ltd and the PROXIDRUGS members Dr. Koraljka Husnjak, Professor Manuel Kaulich and Professor Ivan Đikić, at the Institute of Biochemistry II at the Goethe University Frankfurt, have defined a new class of so-called “intramolecular bivalent glue,” which bind proteins – crucial to the cells that allow our bodies to function correctly – that would otherwise stay apart. This new mechanism binds to two sides of the target protein instead of just one, prompting a rearrangement of the whole protein and stabilising its previously unknown interaction with the E3 ligase.
This has also revealed previously underappreciated properties of molecular glues, paving the way to develop deeper understanding of glues that could allow for new classes to be discovered more rapidly.