28 Jul 2020 - Expanding a toolkit in chemical screening for discovery of novel medicines.
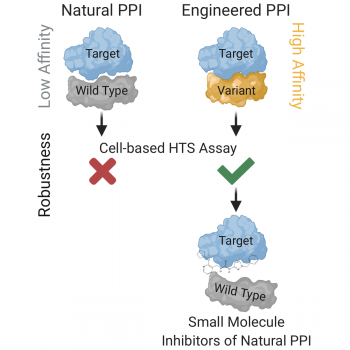
Weak binding affinity between two interacting proteins is very beneficial for cellular signaling cascades, ensuring their dynamic ON/OFF regulation. However, weak affinity of protein-protein interactions (PPIs) also represents a major obstacle in chemical screening. The root of the problem is a lack of approaches for targeting such interactions, ultimately limiting our abilities to discover novel medicines. In an article published in Cell Chemical Biology, Maculins et al., 2020 describe an innovative approach to chemical screening.
The Molecular Signaling group headed by Prof. Ivan Dikic at the Institute of Biochemistry II in collaboration with researchers at the Fraunhofer Institute for Molecular Biology and Applied Ecology approached this obstacle by engineering a high-affinity variant of one interaction partner that maintains binding to the relevant functional epitope on the second interaction partner. This ‘artificial PPI’ enabled generation of a robust cell-based chemical screening platform and identification of high-confidence hit molecules. To validate this novel approach, the team tackled the interaction between the UBAN domain of NEMO and linear ubiquitin chains that plays a key role in NF-κB pathway activation. In this multi-functional drug discovery project, the team identified three high-confidence chemical hits that dampened NF-κB pathway activation through acting on the endogenous ‘natural PPI’ between UBAN and linear ubiquitin chains. By addressing one of the biggest limitations in chemical screening, the research team awaits application of their approach in many drug discovery programs resulting in novel medicines.