News from the Institute
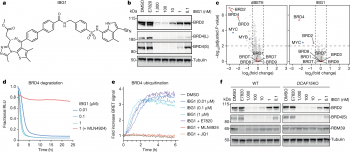
A new class of molecular glue could pave the way for a new generation of drugs to target cancers and neurodegenerative diseases.
... (read more)
A collaborative research project between scientists from Technion - the Israel Institute of Technology in Haifa, Goethe University Frankfurt and Helmholtz Center Munich/Ludwig Maximilian University Munich on “Understanding and Targeting Degradation-resistant Cancers” is funded with 1.6 million € as part of the German-Israeli Project Cooperation (DIP). DIP was inaugurated in 1997 by the German Federal Ministry for Education and Research (BMBF) to strengthen excellence in German-Israeli research cooperation. The Deutsche Forschungsgemeinschaft (DFG, German Research Foundation) has taken over and continues the program while BMBF continues to provide funding for the five-year projects.
... (read more)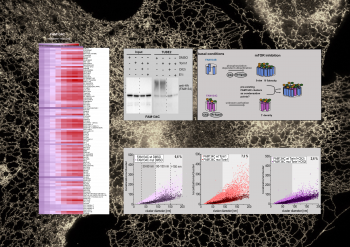
Content and dimensions of the endoplasmic reticulum (ER) are flexible and regulated based on cellular needs, environmental changes or stress. Turnover or degradation of superfluous material is facilitated by selective degradation of the ER via the lysosome (ER-phagy). Malfunction of ER-phagy usually results in disease formation, including neurodegenerative diseases.
... (read more)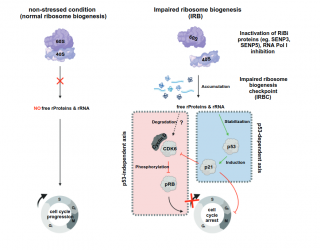
Ribosome biogenesis, a complex and highly regulated cellular process, involves the orchestrated assembly of pre-ribosomal particles to generate functional ribosomes and is coordinated by a network of trans-acting factors. Ribosome biogenesis is closely tied to cell proliferation and highly proliferating cells typically show enhanced rates of ribosome biogenesis to enable them to meet the needs for extensive and efficient protein synthesis. Disruptions can activate a cell-cycle checkpoint mediated by the tumor suppressor protein p53 that block cell cycle progression
... (read more)
On 1 December, IBC2 group leader Christian Münch took up the Lichtenberg Endowed Professorship for Molecular Systems Medicine. His binding to Goethe University Frankfurt was made possible by co-financing from four foundations: The Volkswagen Foundation is contributing a total of €2 million as part of its "Lichtenberg Endowed Professorships" program, with a further €3 million being provided by the Johanna-Quandt-Universitätsstiftung, the Alfons und Gertrud Kassel-Stiftung and the Dr. Rolf M. Schwiete Stiftung.
... (read more)