News from the Institute
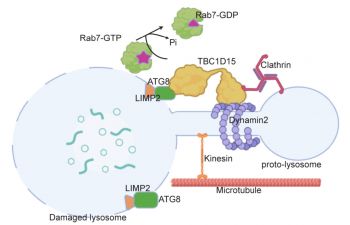
Lysosomes are small membranous organelles which are essential for recycling cellular waste. They are critical to cellular health and lysosomal dysfunction is a predominant feature of several diseases like lysosomal storage disorders, neurodegeneration and nephropathies. The absence of functional lysosomes leads to a build-up of toxic cellular waste which is detrimental to all aspects of cellular metabolism and function.
... (read more)
Ivan Đikić, IBC2 Director and Brenda Schulman, Director at the Max Planck Institute of Biochemistry in Martinsried will together be awarded the 2023 Louis-Jeantet Prize for medicine for their outstanding scientific achievements in the ubiquitin field.

Manuel Kaulich, professor at the Institute of Biochemistry II of Goethe University Frankfurt, was selected as an Henriette Herz Scout for the Alexander von Humboldt foundation. The Henriette Herz Scout program selects internationally recognised scientist, who demonstrated scientific excellence, successfully advanced the career of young scientist, and have large cooperation networks to open up opportunities for attracting international scientific talent. The award allows Manuel to recruit two international postdocs/scientist to his group and support their stay with a prestigious Humboldt fellowship, along a variety of educational and networking opportunities.
... (read more)
For his poster "A high resolution cryo-EM structure of the Mrp antiporter from Bacillus pseudofirmus offers insights into ion transfer pathways" the IBC2 PhD student Anton Altmeyer received the FEBS Letters Poster Award at the European Bioenergetics Conference 2022 which took place from the 20th to the 25th of August in Aix-en-Provence, France.
... (read more)
IBC2 director Ivan Đikić is one of the five new members of EMBO Council that were elected at the Council meeting in Heidelberg on 24 and 25 May 2022. Đikić will join the Council in 2023. The EMBO Council, composed of 15 members, is the governing body of EMBO and as such responsible for developing and guiding the future directions of the organization.
... (read more)