09 Jun 2020 - Uncovering links between SUMO signaling and neurodegenerative disease
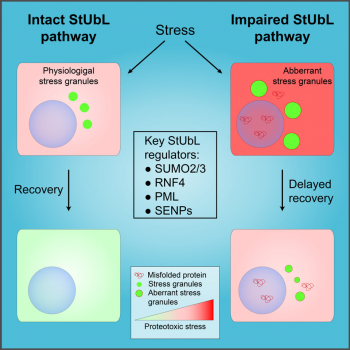
As published in Molecular Cell today, the Müller group has discovered a unique link between SUMO-mediated nuclear protein quality control and cytosolic stress granules (SGs). SGs are ribonucleotide particles that represent a prototypic example of membrane-less organelles. SG assembly is an important stress response that limits protein synthesis to avoid further influx into overloaded proteostasis systems. Typically, SGs rapidly disassemble when stress resolves. This process is impaired in amyotrophic lateral sclerosis (ALS), a neurodegenerative disease, due to mutations in RNA-binding proteins such as FUS or TDP-43. Jan Keiten-Schmitz and coworkers now found that the proper disassembly of SGs upon release of heat or arsenite stress requires both SUMO chain formation and the SUMO-targeted ubiquitin ligase (StUbL) RNF4. Strikingly, they could show that this pathway limits the accumulation of an ALS-causing FUS mutant in SGs. Taken together, these data demonstrate that the StUbL pathway is intricately linked to the dynamics of SGs and might be exploited therapeutically in ALS.