05 Nov 2019 - Novel enzyme class counteracts PR ubiquitination
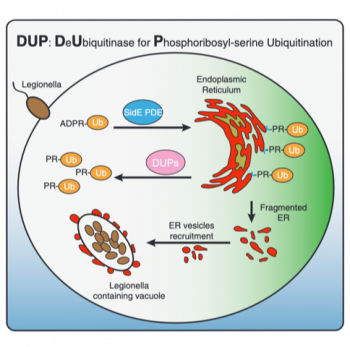
Phosphoribosyl-serine (PR) ubiquitination is a novel type of ubiquitination utilized by Legionella pneumophila during infection. In the past three years, IBC2 Director Ivan Dikic and his team were amongst the front-runners in unravelling the exciting chemistry behind. Now they add a new piece to the puzzle: As reported in today’s online issue of Molecular Cell, they discovered two bacterial effectors which are capable of removing PR ubiquitination. According to their function, they named the proteins as DUPs: DeUbiquitinases for PR ubiquitination. They continued to elucidate the catalytic mechanism of DUPs, providing detailed structural understanding of yet another novel chemistry.
In a next step, they utilized a mutant DUP form to trap and identify PR-ubiquitinated cellular components. This is the first systematic identification of targets for PR ubiquitination, and it will provide unprecedented insight into the host-pathogen interaction upon Legionella infection. Donghyuk Shin, researcher in the Dikic group, comments: “It is surprising how this bacterium uses completely new chemical reactions to attack host cells. Many Legionella proteins remain unknown and need to be explored. We hope our studies provide new approaches to develop novel antibacterial reagents.” Legionella pneumophila causes Legionaries’ disease which presents with an atypical form of pneumonia and may be fatal in elderly and immunocompromised patients.