02 Jan 2015 - Unprecedented insights into proton pumping
Cells convert the energy extracted from foodstuff into ATP, the universal currency of cellular energy. Mitochondrial oxidative phosphorylation is carried out by five large enzyme complexes.
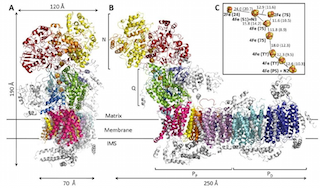
The Zickermann group from Institute of Biochemistry 2 together with colleagues from the Cluster of Excellence Macromolecular Complexes in Frankfurt and from Freiburg University solved the 3D structure of mitochondrial complex I, the largest and most complex enzyme of this fundamental metabolic pathway.
Complex I comprises more than 40 subunits and has a mass of almost 1 Mega-Dalton. Its major task is to pump protons across the inner mitochondrial membrane, thereby driving ATP synthesis. The exact mechanism, however, has remained elusive.
In todays issue of Science, the Zickermann group now reports new and exciting insights into the structure of this giant enzyme complex, explaining how it transmits the energy needed for proton pumping over quite a long range. The team was able to come up with a new model, by which a sequential formation of charged intermediates causes a concerted rearrangement of structural elements within the enzyme, thereby ultimately triggering proton translocation at the pump sites.