10 Jul 2014 - Novel role for ubiquitin in the physical and functional disassembly of a MAPK cascade
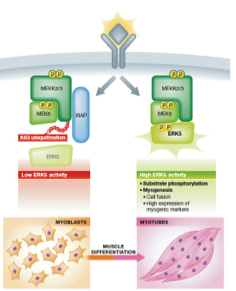
Mitogen activated protein kinases (MAPKs) are a class of highly conserved family of protein kinases that control fundamental cellular processes like cell survival, migration and differentiation. The group of Krishna Rajalingam from IBCII, now discovers a novel role for ubiquitination in the inactivation of MEKK2/3-MEK5-ERK5 signaling pathway, thus adding another layer of regulation of MAPKs. They identify that two members of the inhibitors of Apoptosis Proteins (IAPs) family, XIAP and cIAP1, directly bind and conjugate a unique type of ubiquitin chains to MEKK2 and MEKK3, which directly impairs the complex formation between MEK5 and ERK5 thus inactivating this signalling pathway. They further identify that XIAP negatively controls human myogenic differentiation by regulating the activation dynamics of ERK5-MAPK cascade. The observations by Takeda AN et al are now published in EMBOJ