News from the Institute

Until now, little was known about the identity of proteins which are disposed via autophagy. In an unbiased approach to shed light on this conundrum, the Behrends group used a novel proteomics technology to selectively capture all cargo proteins carried by autophagosomes in living cells.
The outcome was a long list of 1,147 proteins – amongst them numerous involved in mitochondrial function, a rather unexpected result considering that no treatment triggering mitophagy was applied. The researchers followed up on this and made a yet more surprising discovery:
... (read more)
IBC2 group leader Christian Münch receives one of the prestigious Emmy Noether Grants from the German Research Foundation (DFG).
His project aims at deciphering the mitochondrial unfolded protein response (UPRmt) in mammalian cells and will be funded with 2 Mio Euros for up to 5 years. Until now, the signaling network determining the UPRmt has remained largely elusive.
... (read more)
Stefanie Oess was appointed as Chair of Biochemistry at the Brandenburg Medical School Theodor Fontane. Within the still young university, Stefanie will play a critical role in shaping the biochemistry curriculum for students.
In parallel, she will be continuing to push forward her challenging and unique research program in cardiovascular signaling. The Oess laboratory will be situated within newly built premises on Campus Brandenburg, with Meike Hoffmeister as laboratory head overseeing all scientific operations.
... (read more)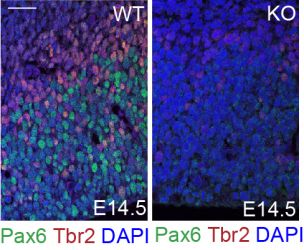
The IBC2 research group of Stefanie Oess recently discovered that the protein Nosip is crucial for the function of neural stem/progenitor cells and thus critical for the complex process of brain development. In previous work, the group had already elucidated that the E3 ligase Nosip is a regulator of forebrain and craniofacial development in mice.
In their latest study, the researchers now combined in vivo and in vitro techniques and applied state-of-the-art proteomics to describe a novel function of Nosip upstream of Retinol-binding protein 1 (Rbp1) in early neurogenesis.
... (read more)
Today, the German Research Foundation (DFG) announced the establishment of a new Research Unit (FOR 2625) on the mechanisms of lysosomal homeostasis.
The Unit is led by Thomas Braulke from the University Medical Center Hamburg-Eppendorf, with 13 research groups from Germany and one from The Netherlands taking part. From IBC2, Anja Bremm and Ivan Dikic are on board, bringing in their expertise in ubiqiuitin signaling and deciphering how ubiquitin regulates lysosome function.
... (read more)